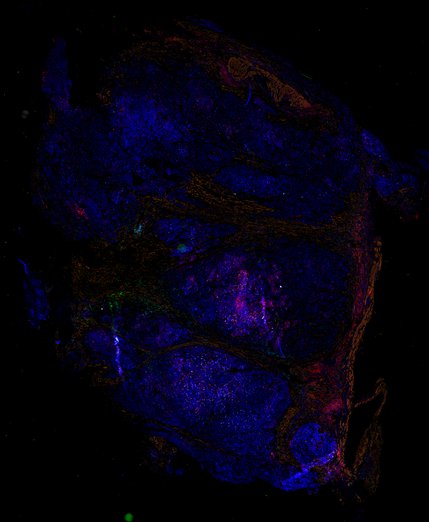
Evolution of Delayed Resistance to Immunotherapy in a Melanoma Responder
David Liu*, Jia-Ren Lin* (equal contribution), Emily J Robitschek, Gyulnara G Kasumova, Alex Heyde, Alvin Shi, Adam Kraya, Gao Zhang, Tabea Moll, Dennie T Frederick, Yu-An Chen, Shu Wang, Denis Schapiro, Li-Lun Ho, Kevin Bi, Avinash Sahu, Shaolin Mei, Benchun Miao, Tatyana Sharova, Christopher Alvarez-Breckinridge, Jackson Stocking, Tommy Kim, Riley Fadden, Donald Lawrence, Mai P Hoang, Daniel P Cahill, Mohsen Malehmir, Martin A Nowak, Priscilla K Brastianos, Christine G Lian, Eytan Ruppin, Benjamin Izar, Meenhard Herlyn, Eliezer M Van Allen, Katherine Nathanson, Keith T Flaherty, Ryan J Sullivan, Manolis Kellis, Peter K Sorger, Genevieve M Boland
Nat Med. In press
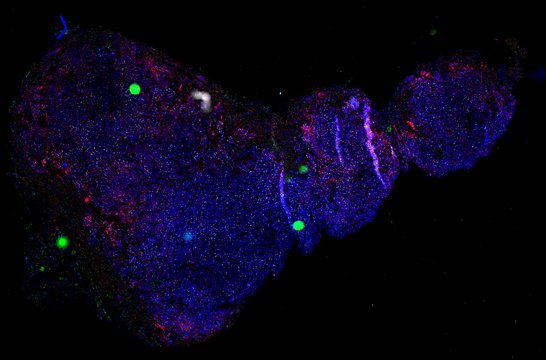
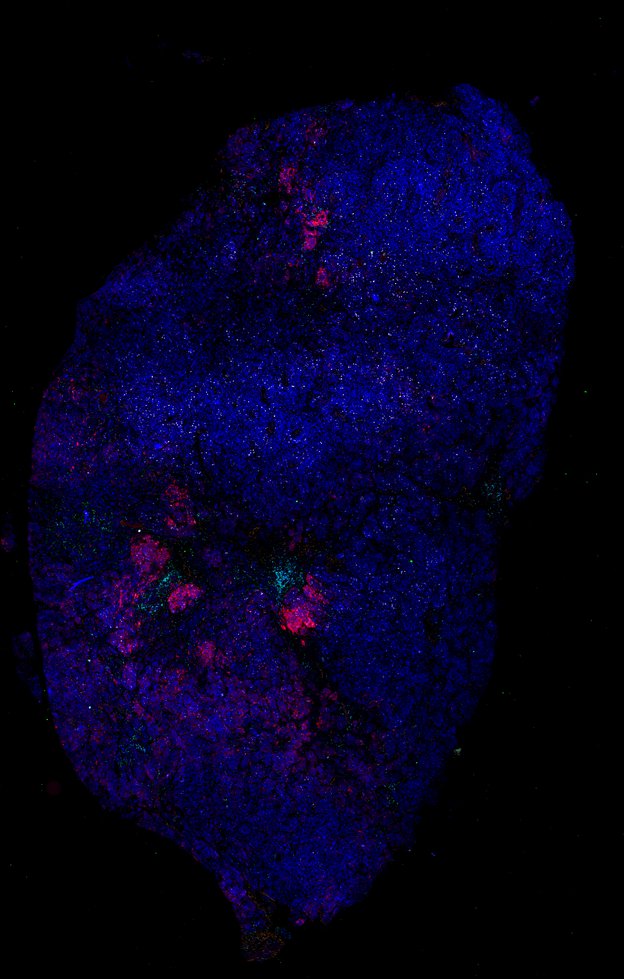
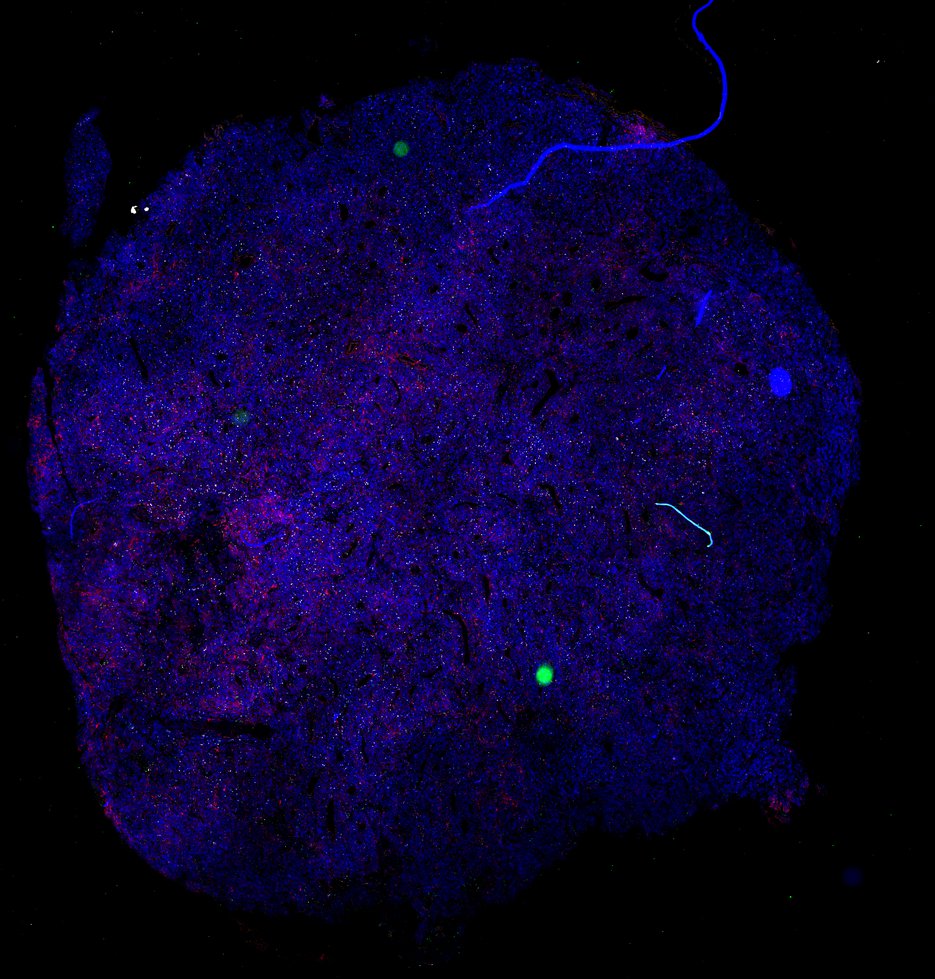
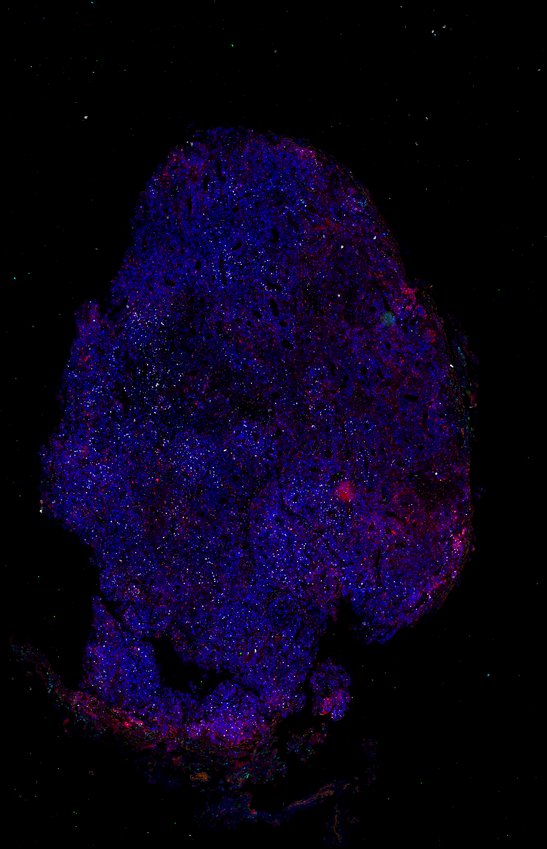
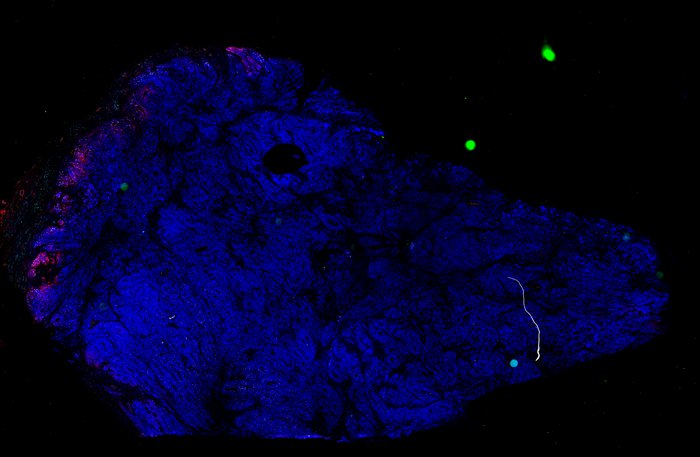
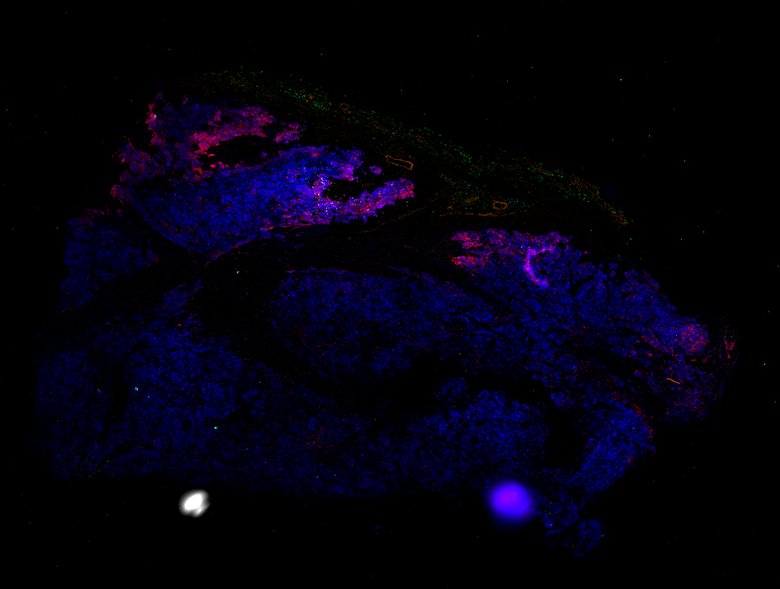
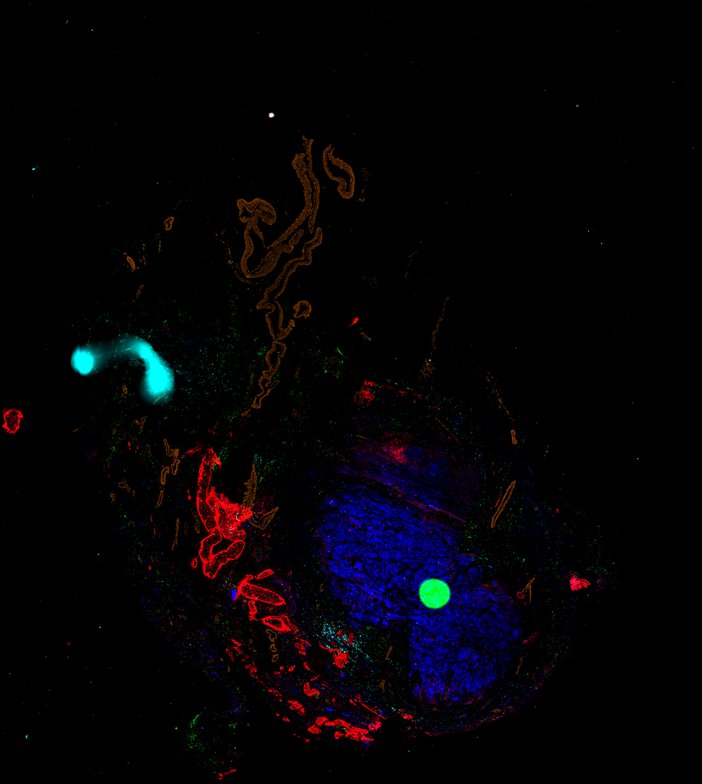
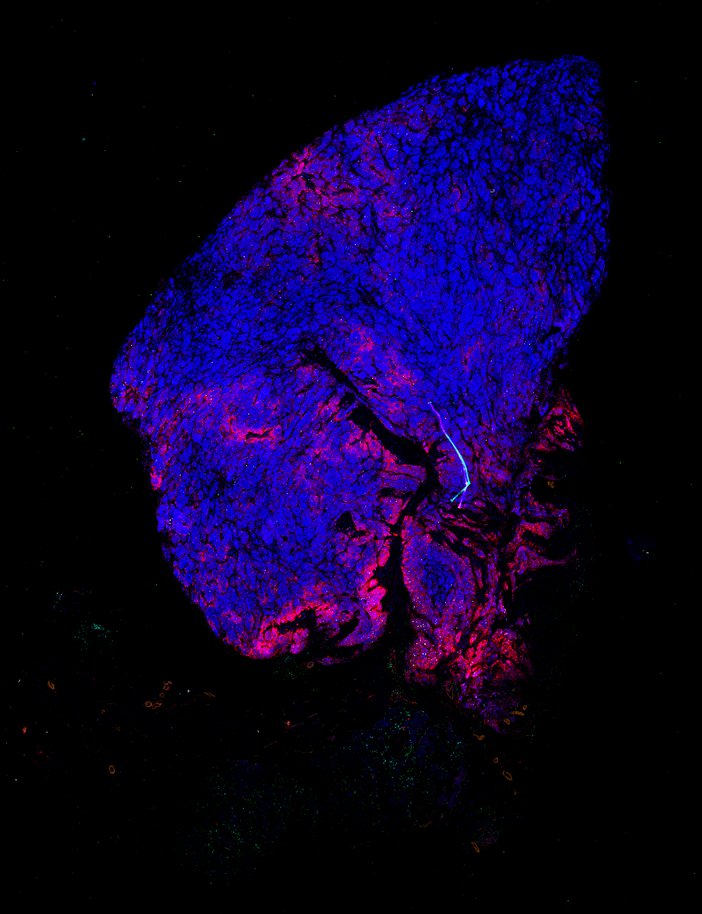
Despite initial responses, most melanoma patients develop resistance to immune checkpoint blockade (ICB) via poorly understood mechanisms. To understand the evolution of resistance, we studied 37 tumor samples collected over 9 years from a patient with metastatic melanoma and initial response to ICB followed by delayed recurrence and death. Phylogenetic analysis revealed co-evolution of 7 lineages with multiple convergent, but independent resistance-associated alterations (RAAs). All progression tumors emerged from a lineage characterized by loss of chromosome 15q including β2 microglobulin (B2M), with post-treatment resistant clones continuing to acquire additional genomic driver events including genome doubling, CDKN2A biallelic loss, and chromosome 11 loss. Deconvolution of bulk RNAseq and highly-multiplexed immunofluorescence (t-CyCIF) of histological specimens revealed differences in immune composition amongst the different lineages, despite an overall low immune composition. Imaging also revealed an NGFR-High tumor population enriched for PD-L1 expression in close proximity to immune cells with a vasculogenic mimicry phenotype. Rapid autopsy samples demonstrated 2 distinct NGFR spatial patterns with increased polarity and proximity to immune cells in subcutaneous tumors versus a diffuse spatial pattern in lung tumors, suggesting differences in the role and etiology of this neural crest-like program in different tumor microenvironments.
Available images
Biopsies
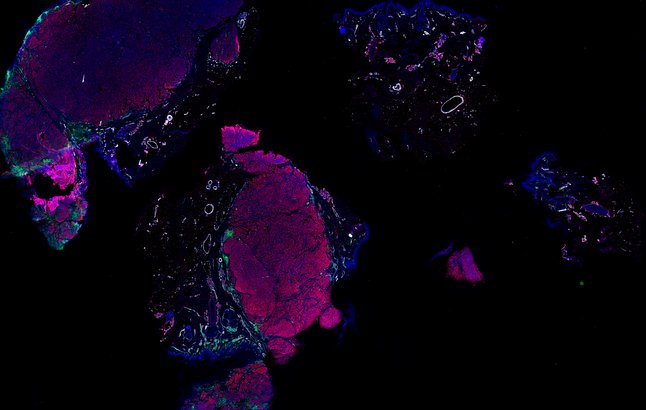
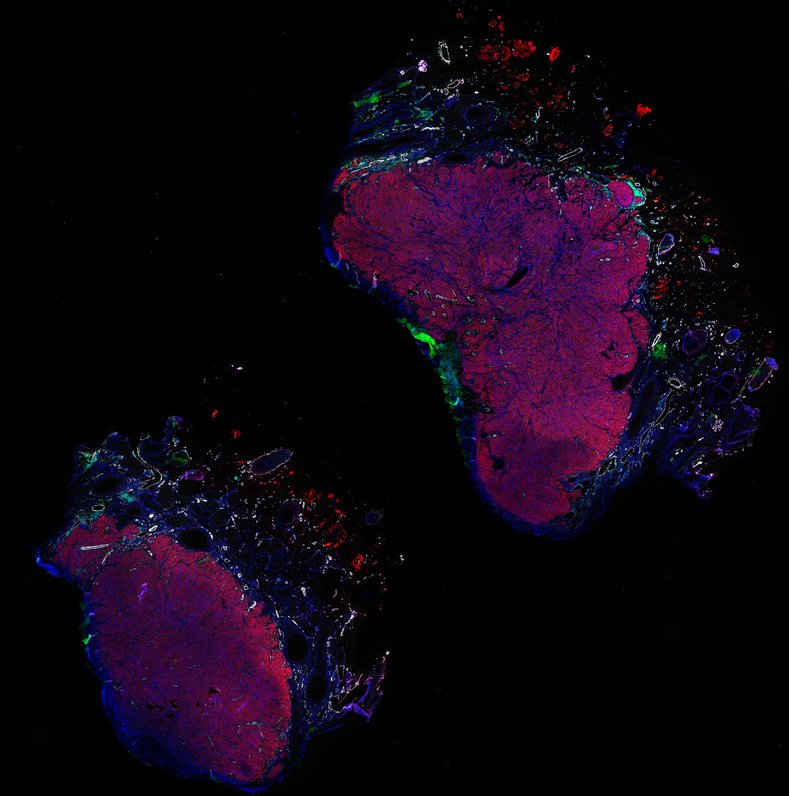
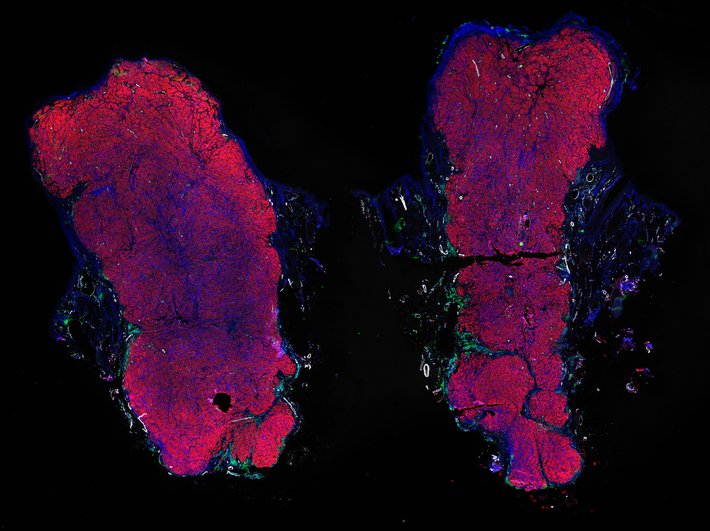
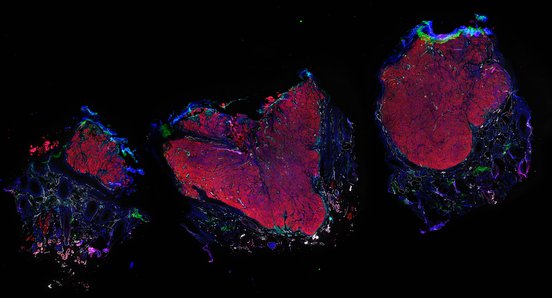
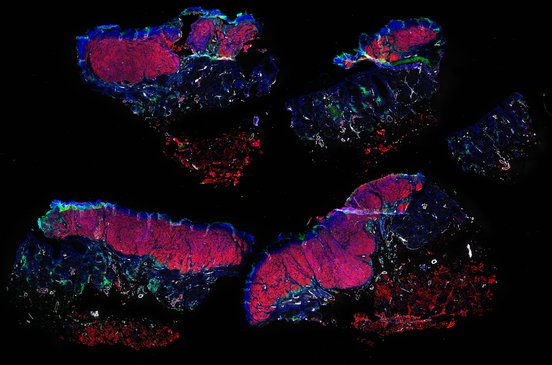
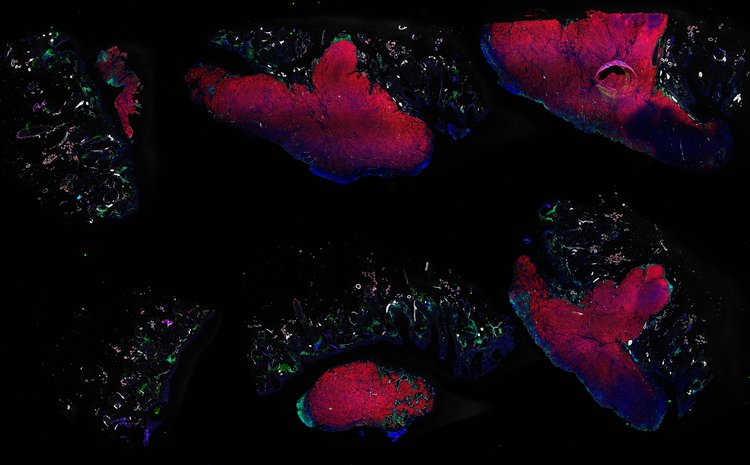
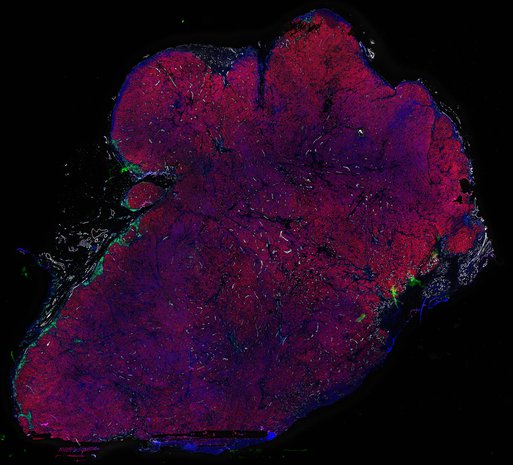
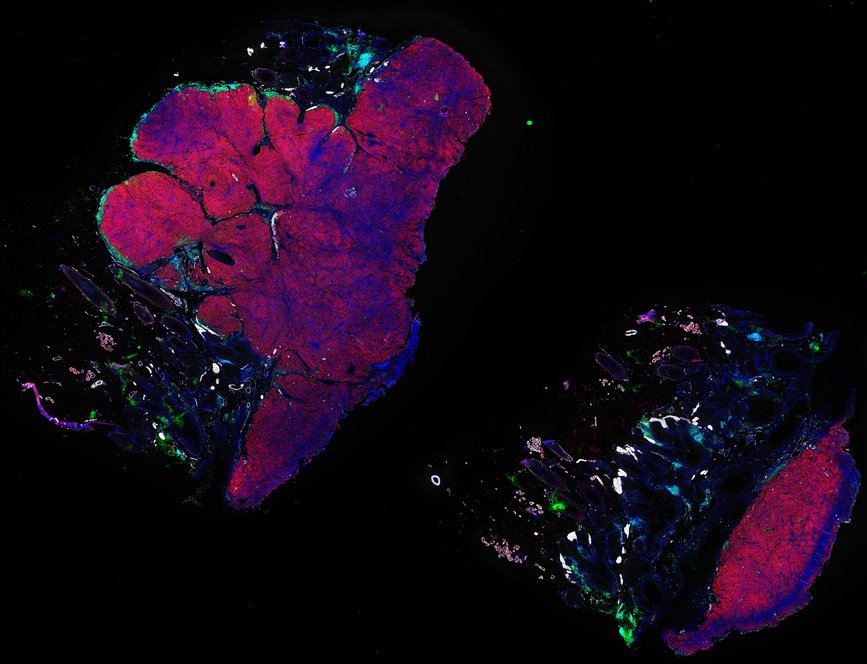
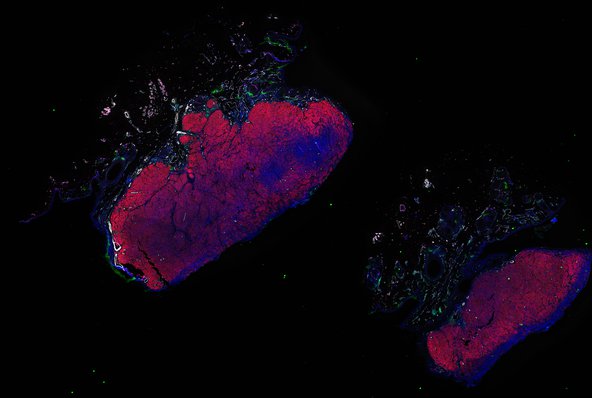
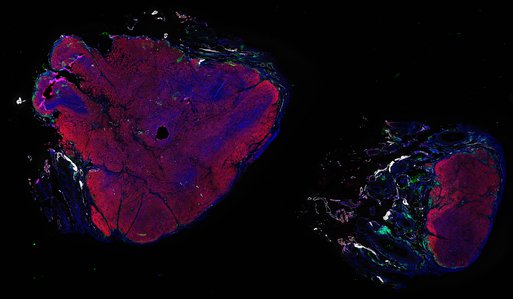
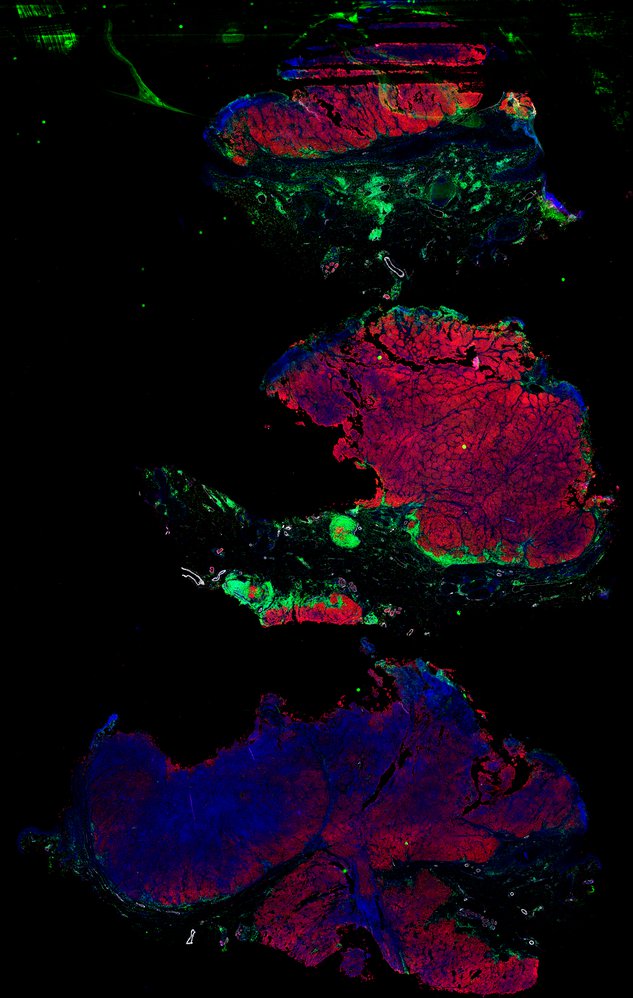
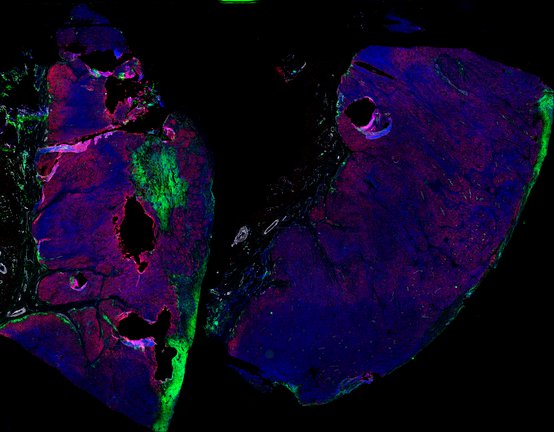
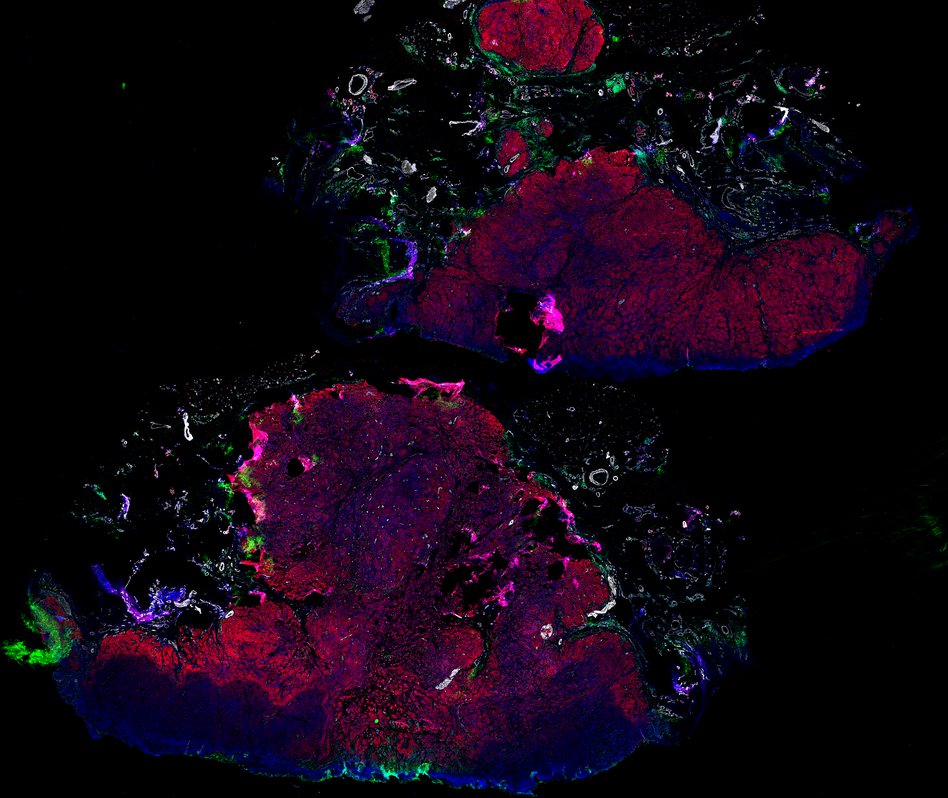
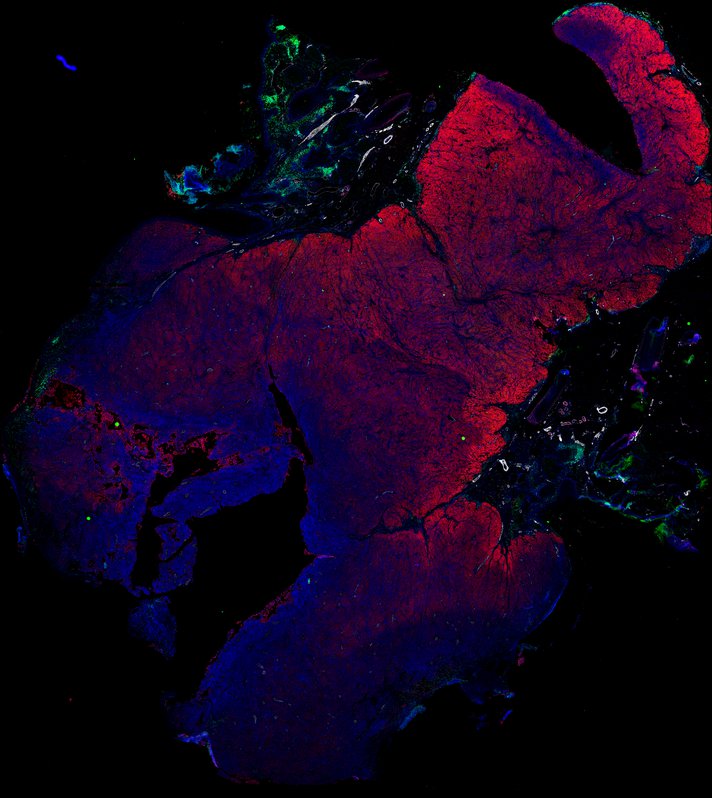
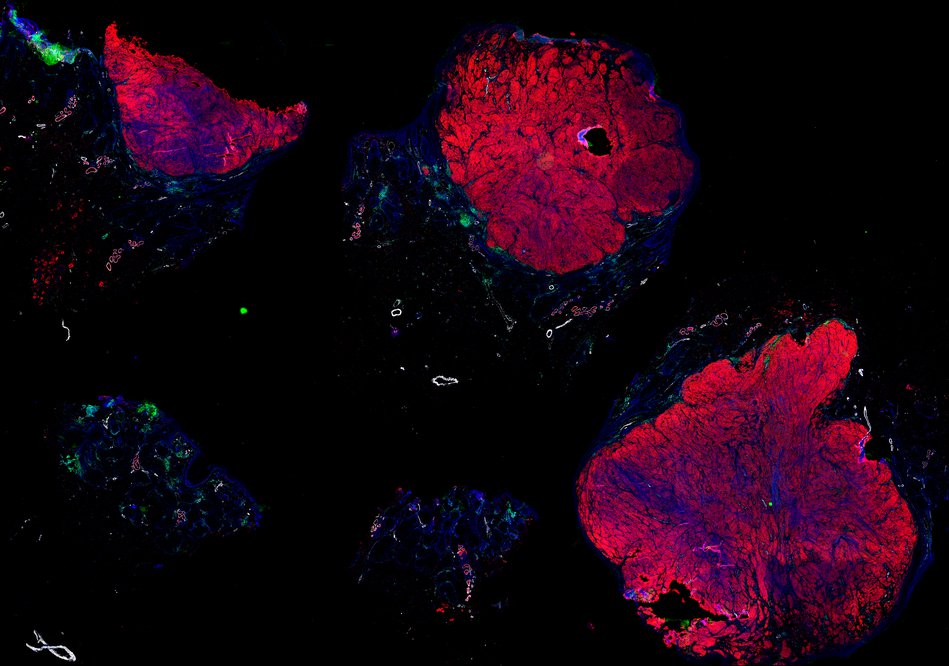
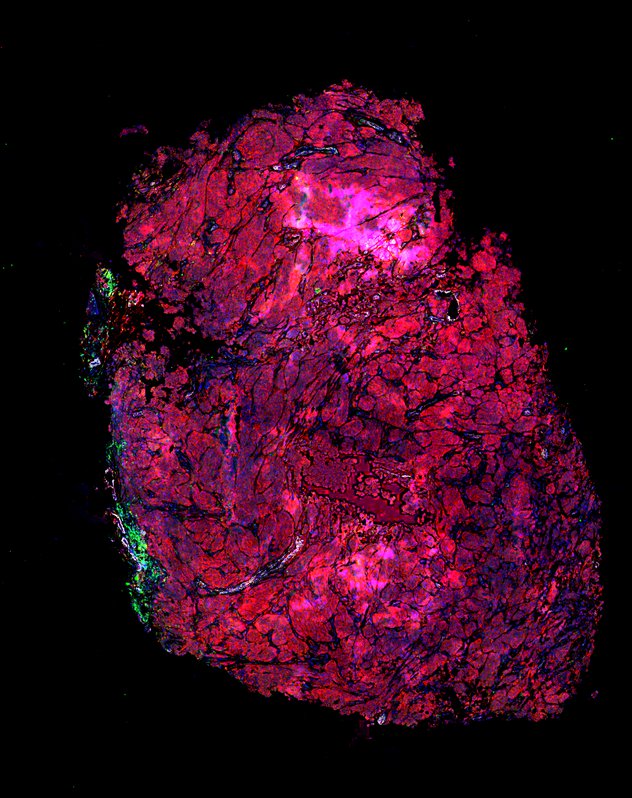
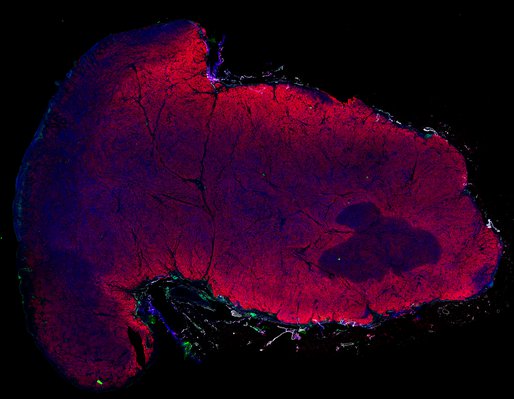
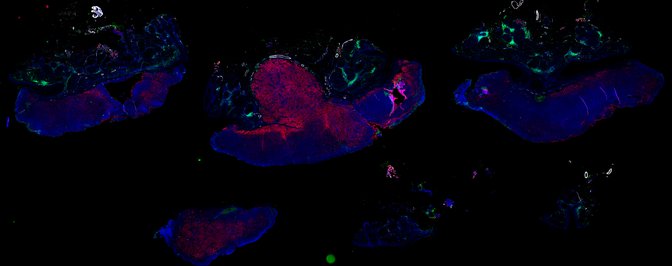
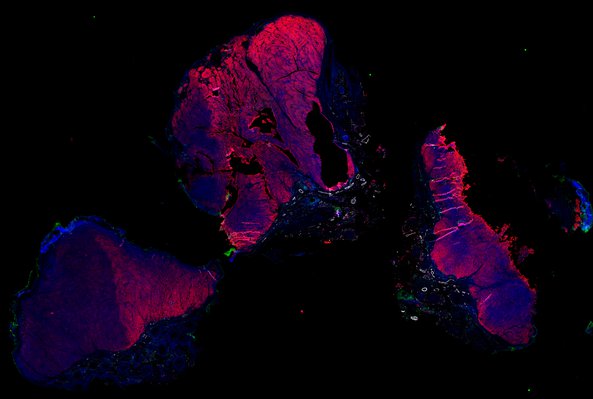
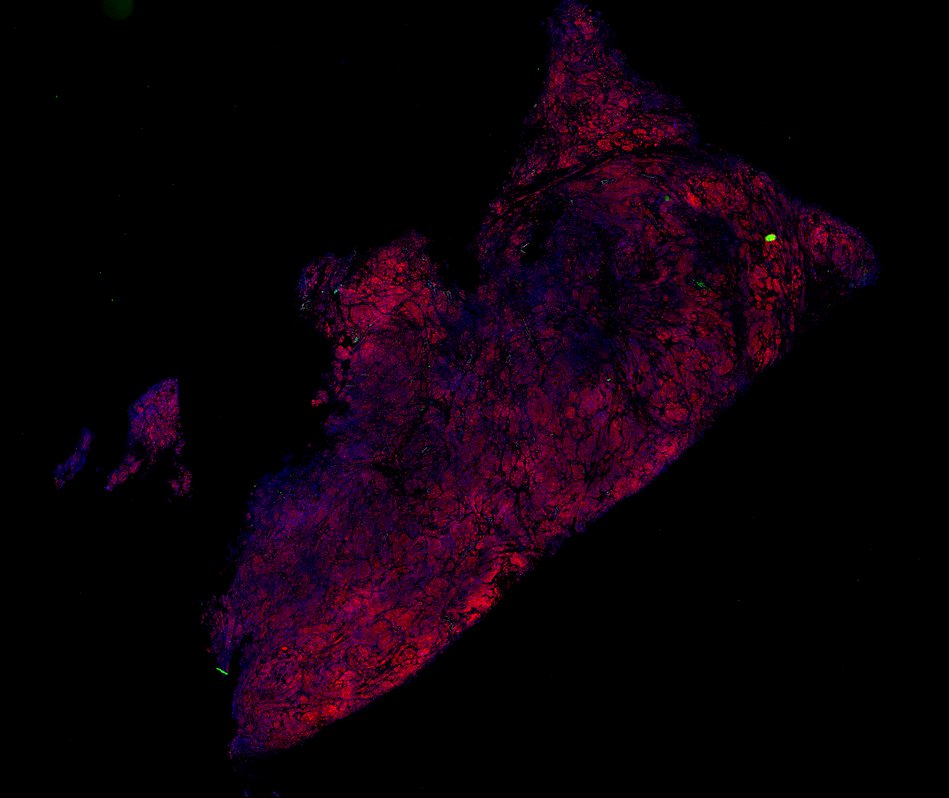
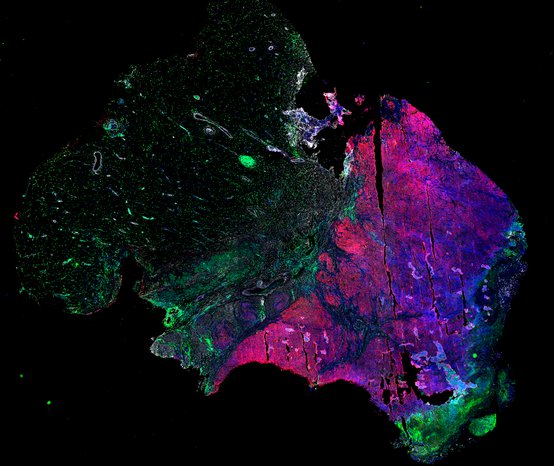
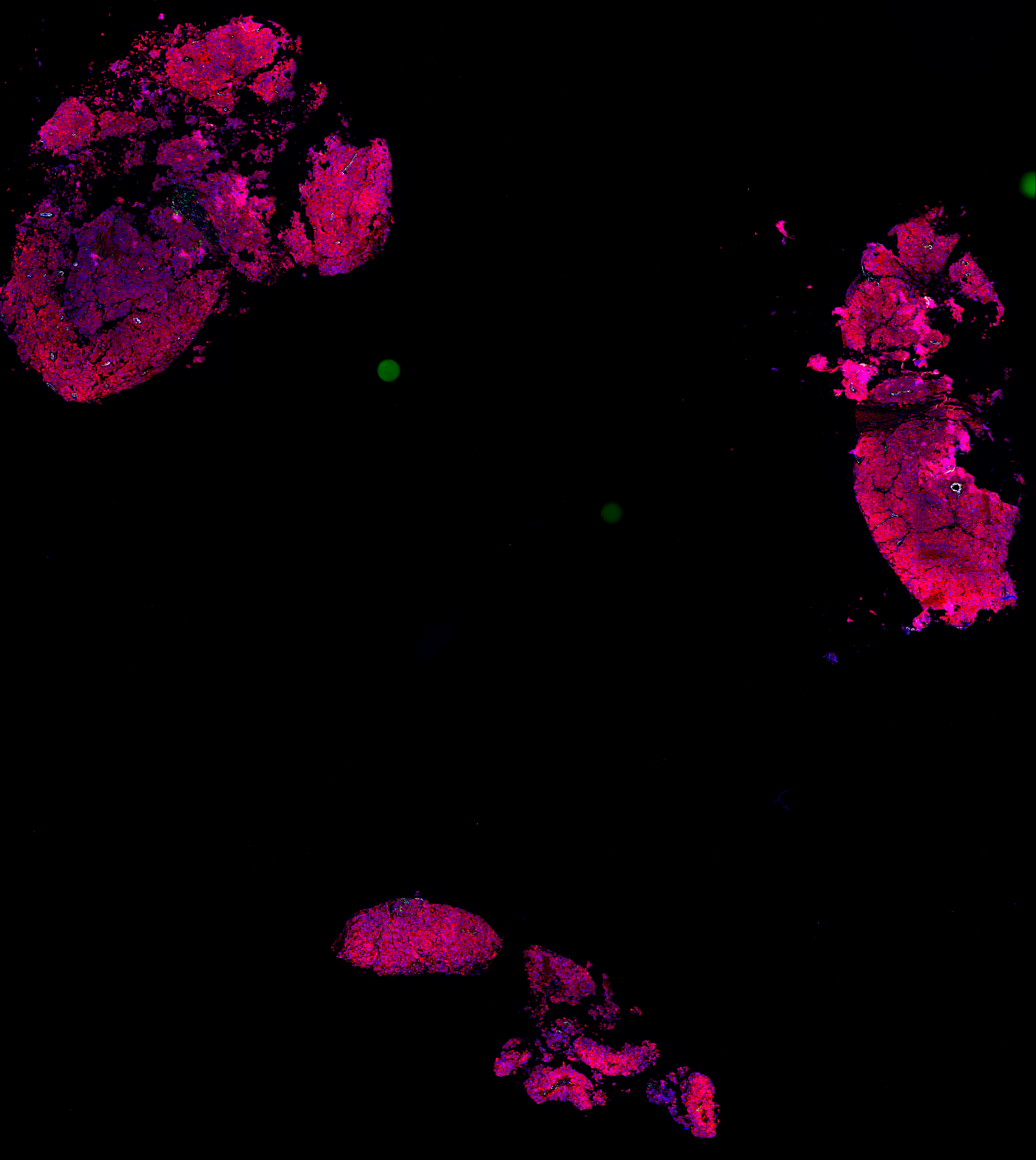
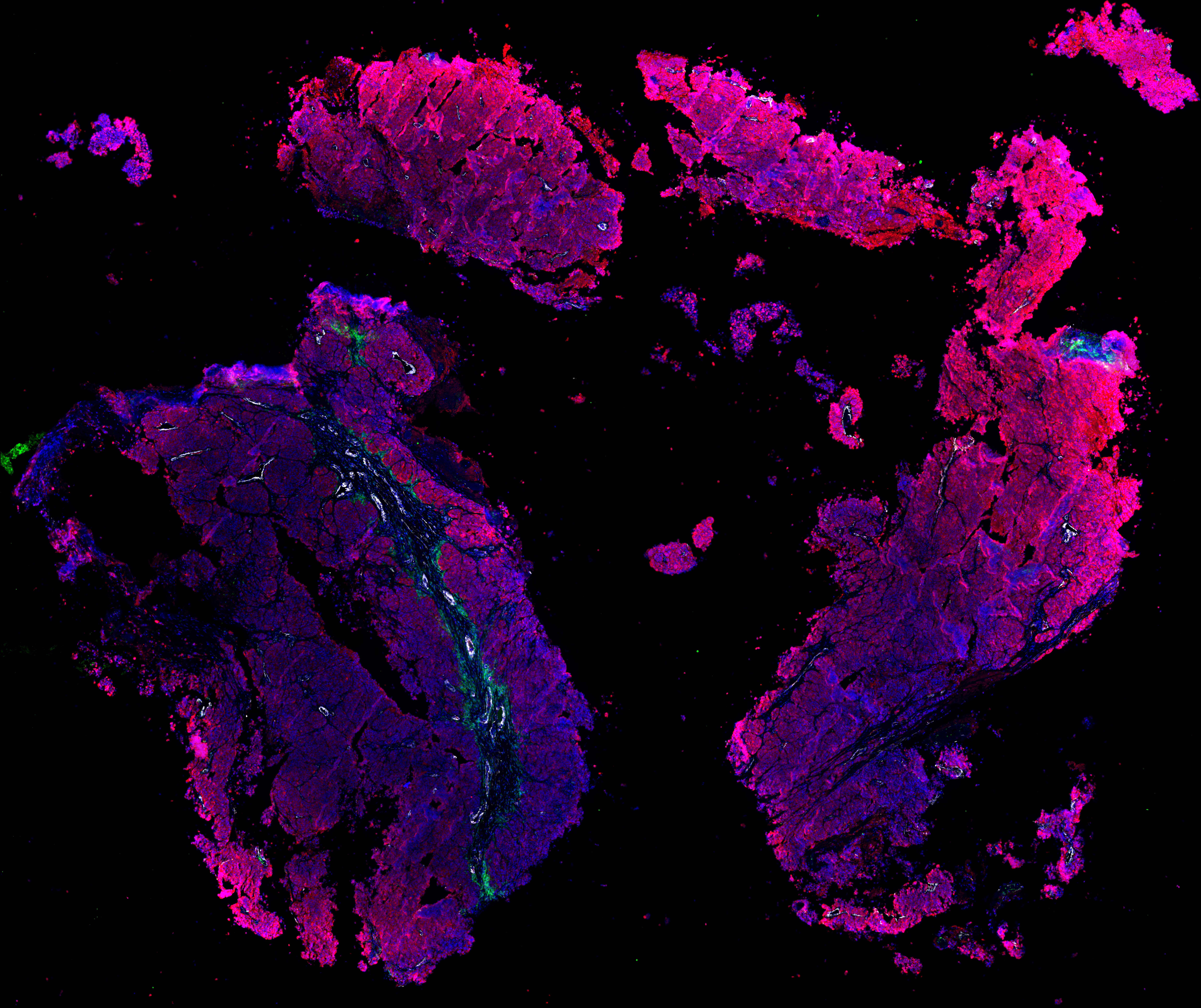
Autopsies